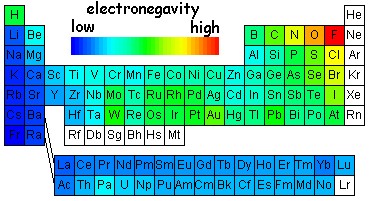
Electronegativity describes the relative pull of the a particular elements on the electrons in a covalent compound. There are several electronegativity scales that have been developed over the years to describe bonding. Some are based on elemental properties (ionization energy and electron affinity) other are based on bond strength measured in various compounds. However, all the scales share the same qualitative idea. The higher the electronegatively the greater the attraction the electrons have for that element. Since the scale is relative (a comparison between different atoms) F is typically chosen as the most electronegative atom and its electronegativity is set at 4.0. Electronegativity has a periodic trend that is nearly identical to that of ionization energy. It decreases top to bottom and it increases left to right. The reason behind this is nearly identical to that of ionization energy. The pull of the nucleus increases left to right and decreases top to bottom. Below is a scale of electronegativities. To calibrate the diagram F = 4.0 and Cs = 0.8

You can see a few exceptions to the strict increases left to right and decreases top to bottom (particularly in the transition elements). However in general, the upper right is the highest and the lower left is the lowest. Another notable outlier is hydrogen. Because hydrogen has only one electron, it is quite different from the other elements. Its electronegativity is nearly identical to that of carbon.