Ionic compounds are compounds that are composed of discrete ions or charged species. There could be elemental ions, atoms that have lost or gain electrons. Or they could be polyatomic ions: a discrete collections of atoms that have a charge. One of the most important ideas regarding ionic compounds is that they are not themselves discrete units. They are collections of thousands, millions, septillions,....of ions. When chemists talk about a water molecule, they are referring to a discrete collection of three atoms, two hydrogen and one oxygen, that are stuck together. You can image a single molecule of water (just those three atoms) floating in space. In fact, this is not a bad picture for water vapor. Ionic compounds are very different. The ubiquitous example of an ionic compounds is sodium chloride, NaCl. It is this huge collection of sodium cations, Na+ and chloride anions, Cl-. However it is not just a single pair of these ions. It is an enormous number of them. The key is that the ratio of the two ions is always one to one. Thus the "formula unit" (note this is not the "molecular formula") is NaCl. One Na+ for every Cl-.
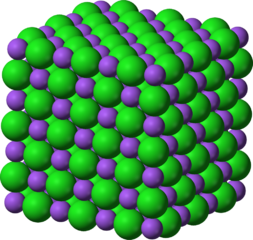
Ionic compounds form large repeating structure of ions. This structure is known as a crystal lattice. It is a specific pattern in which the position of each ion is known in relation to the other ions. Here are two pictures of sodium chloride, NaCl. Each diagram tries to illustrate the relative positioning of the sodium ions (the smaller ones) and the chloride ions (the larger ones).

Each cation (the blue or purple smaller spheres) in the lattice is surrounded by six anions (the green spheres). So the positive charge of the cation is found in an extremely stable location as it is interacting with six adjacent anions. Similarly each anion is surrounded by six cations. As a result the structure is a very low energy configuration for the ions. These pictures merely depict a small part of the overall structure as it repeats essentially forever.
Ionic compounds are usually combinations of metal and non-metal elements. The metallic elements generally form cations and the non-metal elements typically form anions. For example in NaCl, the cation, Na+, is formed by loss of an electron from the group 1 metal sodium. While the chlorine anion, Cl-, is formed by the gain of an electron by the group 7 non-metal chlorine atom. The compound is composed of a one to one ratio of these two ions. Other compounds exhibit different ratios of cations and anions. For example in magnesium bromide, MgBr2, the ratio of cation:anion is 1:2. Magnessium is a group 2 metal that forms cations with a stable noble gas electronic configuration when it loses its two valence electrons. Bromine forms a stable noble gas electronic configuration when it gains one electron. Thus we find magnesium as 2+ cation and bromine as a 1- anion in a 1:2 ratio in the compound. The resulting net charge for the compound should be neutral.
Ionic compounds are not all combinations of metals and non-metals. There are a large number of ionic compounds where one of the ions is a polyatomic ion. This is a collections of atoms that are "covalently bonded" together, but where this collection carries with a positive or negative charge. A list of common polyatomic ions can be found here . For all of the ions on this list you should know both their names and formula.