The structure (what atom is bound to what atom) for covalent compounds is not always easy to infer from the molecular formula. However, the bonding (what is bound to what and the order of the bonds: single, double or triple) is critically important for predicting the chemical and physical properties of the covalent compound. Chemists often use a simple tool called Lewis Structures to help correctly predict the structural formula of these compounds. The guiding principle is that atoms tend to arrange in ionic or molecular clusters to satisfy the octet rule (achieve Noble gas configuration).
Formal charge is a concept used to account for the distribution of electrons in a compound. In a structure, each atom is assigned a formal charge based upon the number of valence electrons for that atom as well as the distribution of electrons in the structure. The formal charge can be calculated as,
\[FC = V - (L + {1 \over 2}S)\]
Where FC is the formal charge, V is the number of valence electrons, L is the number of lone pair electrons, S is the number of shared electrons
For more on formal charge see the "formal charge" page.
FC should be nearest to 0 for all atoms, total FC is 0 for neutral compounds, FC should = charge on polyatomic ion.
Here are a couple of videos working some examples of Lewis dot structures.
Lewis Structures - IntroductionLewis Structure - Ammonia (NH3)
The purpose of the Lewis structure is to accurately predict the bonding in compounds. Sometimes, when assembling a Lewis structure, one finds that a double bond can be placed in more than one location between two like atoms. When this can occur, this is usually an indication that the actual molecular structure is some average structure existing in some state between the available extremes. The average structure is said to exhibit resonance, where the multiple bond is “resonating” amongst the extremes. Experimental evidence supports this theory showing that the bonds that undergo resonance each have a bond energy between the extreme (i.e., less than the strength of a double bond but greater than the strength of a single bond). A worked example showing the resonance structures for SO3 is shown in the following video:
Resonance Structures for Ozone (O3)Here is a quick self quiz.
For a molecule that has resonance structures in which single and double bonds are flipping, if we could do an experiment to measure the bond strength of those bonds...
A. 50% of the time we would measure a single bond and 50% of the time we would measure a double bond
B. every time the measurement would give the same bond strength close to the average of a single and double bond
The second answer is correct. The first answer is a huge misconception in chemistry. Try try try to put this idea out of your mind. It is simply wrong. The reason we draw resonance structures is not because the bonds in the molecule can't make up their minds, but rather because we insist on drawing structures with single and double bonds. The real molecules is not flipping back and forth between these two but is always somewhere in between.
The video below looks at the bond strengths of ozone (that exhibits resonance) and molecular oxygen.
Bond Strengths for Ozone and OxygenThe formal charge is an idea of accounting for the distribution of electrons in an atom. This can help in two ways.
1. It can help us decide which of several Lewis dot structures is closest to representing the properties of the real compound.
2. It can help us envision where there might be regions of positive or negative charge in a molecule.
First, how does it help to decide between different structures? Our general rule is that the best structure minimizes the formal charges. This is because minimizing the formal charges leads to the electrons being most evenly distributed about the different atomic centers in a molecule. Having electrons concentrated in one area will lead to regions of negative charge. The atoms that are now "missing" electrons will be positive in charge. Separating positive and negative charges costs energy and thus we conclude that the lowest energy (best structure) would minimize having separated charges.
How do we find these charges? We look at how many valence electrons the atoms "has" in the molecule compared to how many it has on its own. It is important to know that this is a very general idea that grossly over simplifies the quantum mechanics. The electrons in a molecule have no memory of where they came from or to which atom they "belong". They are simply spread throughout the molecule. None the less, these simple ideas can help us to arrive at the best structures as well as understand something about the charge distributions.
In a molecule, we assign each atom a formal charge. This charge is the number of electrons it had as valence electrons minus the number it "has" in the molecule. The number it has in the molecule is a combination of the lone pair electrons and the shared bonding electrons. For each atom, we will count all of the lone pair electrons but only half of the bonded electrons (as they are shared). This is easiest to account for by just counting the number of bonds. So the formal charge is
Formal charge = Valence Electrons - [Lone Pair Electrons + (# of Bonds)]

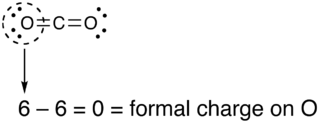
So for example if we look at CO2 each oxygen has two lone pairs (4 electrons) and 2 bonds (double bond). Oxygen has 6 valence electrons. So the formal charge on each oxygen atom will be 6-(4+2)=0.
You need to find the formal charge on each atom in a compound. However, there are some short cuts that will help. The sum of all the formal charges must be the charge on the molecule. For neutral compounds this will be zero. Since each oxygen in CO2 is zero, and the total charge is zero, the formal charge on carbon must be zero.
There are certain situations in which a stable molecule can form containing atoms of elements that are capable of existing with an expanded valence shell (forming bonds in a way that there are more than 8 electrons in the valence shell). When this occurs the compound is said to exhibit expanded valence. This can occur (but doesn’t always occur) for certain elements in period 3 and greater.
You can often identify expanded octet by the S = N-A rule. When this "rule" suggests that you need fewer bonds than needed to put together the skeleton structure, then you need expanded octet. In particular, you'll find expanded octet in odd compounds made from the larger noble gases as well as some of the halogens (in period 3 and greater).
Lewis Structures for Expanded Valence MoleculesThere are certain atoms of certain elements that can exist in stable compounds forming bonds with less than eight valence electrons. When this occurs, the atom of the element within the molecule is said to contain an incomplete octet. The common examples of such elements are hydrogen (stable with only 2 valence electrons), beryllium (stable with only 4 valence electrons) and boron and aluminum (stable with only 6 valence electrons). For hydrogen 2 valence electrons give it a noble gas structure (like He) so this is much like the octet rule for everything else below period 1. But covalent compounds in groups 2 and 3 can form stable compounds in which the valence electrons are not in the noble gas structure. However, for these compounds you will find that they do form compounds that minimize formal charge.
The exceptions are fairly straight forward to remember. Hydrogen makes one bond (group 1). Beryllium makes two bonds (it is the only element in group 2 to make covalent compounds). The group 3 elements boron and aluminum make three bonds. How many bonds do carbon (and the other group four elements typically make)? Four. Why do these trends generally hold? The elements in the same group have similar electronic structure and thus have similar chemistry.
The following video highlights these three exceptions to the octet rule.
Lewis Structures and Incomplete Octets