When we look at trends in the periodic table, we see a clear pattern in which there is a huge change in properties between the noble gases and the alkali metals. This is despite the fact that they are different by only one electron and one proton. From this we arrive at the idea that we can think of the electrons in an atom as being "layered" into shells as we fill up different n levels. The noble gas configurations are the filled shells and represent the lowest energy most stable configurations. After this, the next electron is forced into a higher energy orbital. This alone would not account for the dramatic difference we see because the energy difference between say a 2p electron in Neon and a 3s electron in Sodium shouldn't be that large unless there was another effect. This other effect is the idea of electronic shielding. The idea of shielding is simply that this outer most electron is shielded from the full nuclear charge because of the negative charges of the other electrons "between" it and the nucleus. In this way, we divide up the electrons between those in the stable nobel gas "core" and the new "valence" electrons outside this core. The valence electrons now experience a reduced or effective nuclear charge, Zeff < Z. One way to crudely estimate Zeff is to simply imagine that the core electrons are canceling out the charge of the nucleus so Zeff= Z - (# or core electrons). This over estimates the shielding (as this is the maximum one would every expect) and results in a Zeff that is too small. Never the less, it is good enough to predict the trends.
Here is an illustration showing how to "calculate" +5 as the effective nuclear charge (Zeff) for phosphorus.
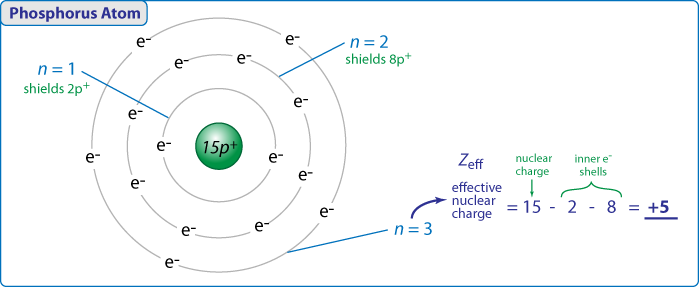
We are not implying that the electrons are in orbits here, this is simply showing inner shell electrons that shield the outer valence electrons from the full nuclear charge. This is a simple picture for us to try to visualize what are actually complex quantum mechanical behaviors. None the less, this simple idea offers good physical insight into the trends we observed in the periodic table. Moreover, this is a simplification of the idea of "shielding". More accurate attempts to quantify shielding would put the Zeff at 5.64 for the 3s orbitals and 4.89 for the 3p orbitals. You can see our hand wavvy guess is not too bad in this situation.
Shielding and Effective Nuclear Charge