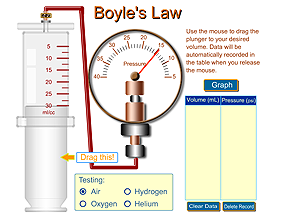
click image to launch applet
Boyle's Law states that the pressure (P) of a gas is inversely proportional to the volume (V). This law is valid as long as the temperature and the amount of gas are constant. Any units will work here:
\[P\,V = k \hskip12pt{\rm (constant)}\]
The constant, \(k\), will depend on the number of moles and the temperature. As long as those two state functions are constant, \(k\) will be a constant and Boyle's Law will hold. Below is a plot of pressure vs volume (aka: a PV plot). Note the shape of the plot, this is a classic inverse relationship.
Most Boyle's Law problems have an initial set of conditions (P1 and V1) and then a final set of conditions (P2 and V2). BOTH conditions must satisfy Boyle's Law and therefore:
\[P_1V_1 = P_2V_2\]
Any units will work here for pressure and volume - just make sure the units are the same on each side of the equation.
Below is a Boyle's Law applet like the syringe we played with in class that allows you to explore the relationship between pressure and volume along with a graph of the data.