The ideal gas law is a simple model that allows us to predict the behavior of gases in the world. It is a combination of the previous laws that we have studied (Boyle's, Charles', Avogadro's). Rather than simply looking at proportionalities, it relates pressure, volume, absolute temperature, and the number of moles quantitatively with a universal constant \(R\) that we call the ideal gas constant.
\[P\,V=nR\,T\]
The value for R will depend on what units you are using for the properties of the gas. The ideal gas law has many implications that will be discussed below. However, the most remarkable aspect is that the same model works quite well for all gases (N2, He, C3H8, ...).
The ideal gas constant is a Universal constant that we use to quantify the relationship between the properties of a gas. The constant \(R\) that we typically use relates pressure in atmospheres, volume in liters, and temperature in Kelvin. In this case, it has the value and units of
\[R=0.08206 {\rm \;\;L\;atm\;mol^{-1}\;K^{-1}}\]
The gas constant \(R\) will appear in many contexts as this is a Universal constant that relates energy and temperature. A pressure times a volume is an energy. As such, you will also encounter the gas constant \(R\) in typical energy units of Joules
\[R=8.314 {\rm \;\;J\;mol^{-1}\;K^{-1}}\]
Finally, ignore the blunder in the video where Dr. Vanden Bout can't remember the correct value for \(R\) with units of atm. The correct value is 0.08206 atm L mol-1 K-1
Often in the laboratory we would like to have conditions (state functions) that are similar to those used by scientists in other laboratories. As such, as a community, we have decided upon some conditions that we will call "standard". The two state functions we can typically control are temperature ( \(T\) ) and pressure ( \(P\) ), so these are the conditions we standardize. We will call these conditions "Standard Temperature and Pressure" or STP. We will take standard pressure as 1 atm, and standard temperature as 0°C. You should note that these are choices. Like many things in life it is difficult to get everyone to agree. Recently, the International Union of Pure and Applied Chemistry (IUPAC) adopted 1 bar as standard pressure and 25°C as standard temperature. This "new" standard is referred to as Standard Ambient Temperature and Pressure (SATP). However, as this new condition has been slow to catch on, we will stick with the "old" standard (STP) unless told otherwise.
Keep them Straight
STP : 0°C (273.15 K) and 1 atm ←(our default "standard" for gases)
SATP : 25°C (298.15 K) and 1 bar
Number density is a useful concept for thinking about macroscopic samples in a microscopic way. Chemists often try to "visualize" materials with a molecular perspective. Number density can be thought of as the number of particles that are present in a particular volume. As these numbers can be very very large, we typically think of this as the number of moles (a fixed number of particles) within a given volume. This quantity is
$${n\over V}={P\over {RT}}$$
So, if we have any two gas samples that are behaving ideally, they have the same number of particles per volume when the temperature and pressure are the same. For example, if I had two balloons in a room, they would have the same pressure (approximately one atmosphere) and the same temperature (whatever the temperature was in the room). Therefore, they would have the same number density. If the balloons had the same volume then they would have identical numbers of particles. This is really a different way of stating Avogadro's Law.
Consider the following diagram... it consists of 3 identical containers (equal volumes) that each contain identical numbers of gas molecules (also assume the temperature is constant). This is illustrated by showing the same number of circles representing the gas molecules.
Even though the particle sizes are different for each case (think about a real case with helium, neon, and argon), the pressures would all be the same. Why? Because number density is what governs pressure for a gas system where temperature is constant. The number densities here are all the same, 10 particles per unit of volume. However, the particle masses are going to be different and therefore the mass density (see upcoming section) will be different for each gas. Specifically, the larger or more massive the particle, the larger the mass density. So the largest mass density is on the right end and the smallest mass density is on the left end.
It is important to always know whether someone is speaking about number density (mol/L) or mass density (g/mL or g/L). They are definitely related, but they are not the same.
Molar volume is another way to view number density. The molar volume is the volume of 1 mole of substance. It can be simply found by dividing the volume of a sample by the number of moles of that sample
\[V_m = {V\over n}\]
This is simply the inverse of the number density. It is a useful quantity to "think about" things from a molecular perspective. As all gases that are behaving ideally have the same number density, they will all have the same molar volume. At STP this will be 22.4 L. This is useful if you want to envision the distance between molecules in different samples. For instance if you have a sample of liquid water, it has a mass density of 1 g mL-1. Since water has a molecular weigh of 18 g mol-1, 1 mole will have a mass of 18 g. From the density this should have a volume of only 18 mL. Clearly this is a lot less than an ideal gas at STP which is more than 1000 times larger in terms of its molar volume.
In the video given below, Dr. LaBrake talks about the Standard Molar Volume of an Ideal Gas.
The mass density of a gas is typically just called the "density". This is the mass of the gas relative to the volume of the gas.
\[{\rm density={mass\over volume}}\]
Because gases that are behaving ideally under the same conditions (temperature, pressure) all have the same number density, they will all have different mass densities since different gases have different masses per particle.
Because we know the number of particles of gas (number of moles) under a given set of conditions, if we measure the gas density, then we can determine the mass of the particles. This is a means by which we can use the density of a gas to determine the molecular weight of a gaseous compound. The density is the mass divided by the volume. Plugging in the volume (\(nRT/P\)) from the ideal gas law we get
\[\eqalign{ {\rm density}&= {m \over V} \;\;=\;\; m \left({1\over V}\right)\cr &= m \left({P \over nRT}\right)\cr &=\left({m \over n}\right)\left({P\over RT}\right)\cr &= MWt \left({P \over RT}\right) }\]
where \(MWt\) is the molecular weight of the compound (\(m/n\)) in g/mol. Using this idea, we can either find the density of a gas given its molecular weight (and the conditions) or use the density (or mass) to find the molecular weight.
\[ MWt = {{\rm density}(RT) \over P}\]
Because the ideal gas law relates all the properties of a gas along with the number of moles of that gas, if we have a measure of the pressure, temperature, and volume of a gas, we actually have a measure of the number of moles of that substance.
\[n={P V\over{R T}}\]
Thus in the same way that we can use the mass of a sample and it molecular weight to determine the number of moles in a particular sample, we can use the temperature, pressure, and volume of a gas to determine how many moles we have.
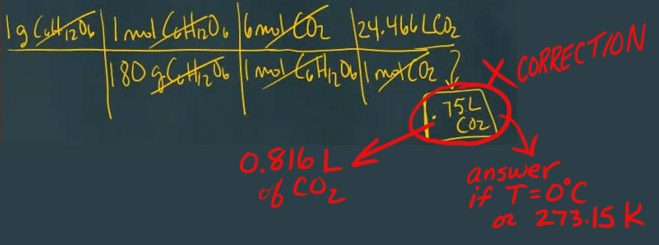
The CORRECT answer in this video is 0.816 L of CO2. Answer shown is the answer IF the temperature was 0°C or 273.15K.
Correction is shown below...