We lump together the attractive forces between molecules, atoms, and ions as intermolecular forces (IMF). IMF is a general term for the attractions between discrete chemical units that we toss together under the general term "molecular."
All of these forces are driven by electrostatics, the forces felt between charged particles. In molecules these forces arise due to the electrons in the molecules.
We have three broad types of IMFs that we will deal with.
Dipole-Dipole forces. These are forces between polar molecules (a molecule with a permanent dipole moment, μ).
Hydrogen Bonding. This is a special case of extreme dipole-dipole forces that occurs when a hydrogen atom is bonded to a highly electronegative atom. The highly electronegative atom has to be N, O, or F.
Dispersion Forces. This one gets several names: Induced dipole-induced dipole, Dispersion forces, London forces, or van der waals forces. Dispersion Forces are often called the "weakest" but it is actually the most important since it is ubiquitous. Every molecule has dispersion forces. Not only do they have these forces, but they can add up across the whole molecule to be substantial. The degree to which a molecule has dispersion forces is measured via its polarizability, α. Polarizability (α) is to non-polar compounds, what dipole moment (μ) is to polar compounds.
Below is a summary of the range of IMFs as they relate to covalent bonds
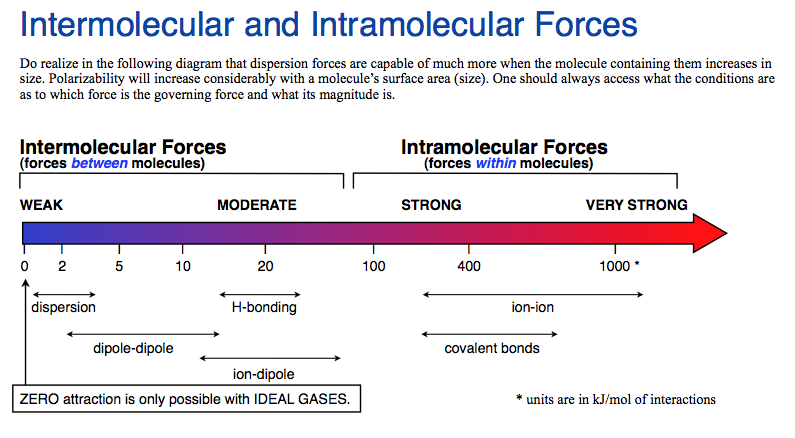
Here is a pdf of this diagram as a helpsheet.
Here is a video of Dr. McCord discussing intermolecular forces.
Intermolecular ForcesA key force of attraction between molecules is the dipole-dipole force. This occurs for polar molecules as they have a permanent dipole moment. You'll remember that a dipole is defined as two opposite charges separated by a distance. Therefore, a molecule with a dipole has a "positive end" and a "negative end." When two or more such molecules come together, they arrange themselves to maximize the attractive forces (negative near positive) and minimize the repulsive forces (like charges). Dipole-dipole forces can be strong and have a long-range interaction. The distance dependence of a dipole-dipole energy scales as 1/r3. Below is a schematic of an arrangement of a polar liquid. Note that there will always be some repulsive interactions, but in general the molecule will arrange to favor the attractive forces (lower energy).
Hydrogen bonding is a very special intermolecular force that occurs in polar molecules when a hydrogen atom is bonded to a highly electronegative atom. The only possible atoms with a high enough electronegativity are N, O, and F. When a hydrogen is bonded to one of these, the sole electron on the hydrogen is shared to such an extent that the hydrogen atom has a much smaller radius. This allows it to approach very close to the N, O, or F atom on another molecule. Since the energy of attraction depends on the separation distance, this particularly close approach leads to a very low energy (very stable) configuration. Thus the effect of hydrogen bonds can be very large. They are so strong that they seem like weak covalent bonds. Thus, the name hydrogen bond. Below is a graph of the boiling point of some compounds like CH4, NH3, H2O, and HF as a function of row in the periodic table with the non-hydrogen element being replaced with every higher mass elements moving down the periodic table. Note the general trend that can be seen in the methane series (CH4, SiH4,....) as the compounds have larger and larger central atoms with more electrons farther from the nucleus they are more and more polarizable. Thus their boiling points increase due to increasing dispersion forces. However, note the clear exceptions for NH3, HF, and H2O. These are the only three compounds that can form hydrogen bonds. This gives them much stronger intermolecular interactions than might be expected from dipole-dipole and dispersion forces alone. Thus they have much higher boiling points than the trend would predict. You can also see that CH4, which has no hydrogen bonds, is not an exception and follows the trend to have the lowest boiling point in the series.
Boiling Points of the Covalent Halides
To start with, dispersion forces have many equivalent names. They are sometimes called induced-dipole induced-dipole forces, London Forces, London Dispersion forces, or van der Waals forces.
They are all names Chemists use to describe the same ubiquitous electrostatic attractive force. To have dispersion forces, a molecule must have electrons. Since all molecules have electrons, they all exhibit dispersion forces to some extent. Dispersion forces are induced-dipole induced-dipole forces that arise from fluctuation in the arrangement of the electrons around a molecule. Even non-polar molecules will not have a perfect distribution of charge for every instant of time. If there is a fluctuation that leads to the molecule having an instantaneous dipole, this dipole will induce a dipole in a neighboring molecule. This will in turn induce another in another neighbor. The induced dipole effect will propagate throughout the whole system. A measurement of this tendency is called polarizability and is given the symbol alpha, α.
As you look through the volume of an actual molecule on a point-by-point basis, dispersion forces will be quite small. However, since this force is everywhere throughout a molecule, the sum of all the interactions can be quite large. As a result, non-polar molecules with no dipole-dipole interactions can have much stronger IMF than polar molecules that have both dispersion and dipole-dipole attractions - so yes, size matters.
While not always "weak," dispersion forces are always very short-range. The energy for a dispersion interaction falls off as 1/r6. Thus the ability for molecules to pack tightly together has a huge effect on dispersion forces. Those that can get very close to one another have much stronger interaction than those that cannot. So once again, the three-dimensional geometry of the molecule has a great affect on its physical properties.
Finally, the polarizability has a larger effect on dispersion forces. The more electrons a molecule has and the farther they are from all the nuclei will affect how easily a dipole can be induced in the electron cloud (aka: polarized). This is why we perceive a molecular weight effect in boiling points. It is not that the mass of the molecules matters, it is simply that more mass implies more protons which implies more electrons. More electrons generally lead to more dispersion forces. Higher mass can also be farther down the periodic table. This will also be more polarizable. A great example of this is the diatomic halogens. F2 is a gas, Cl2 is a gas, but Br2 is a liquid, and I2 is a solid. All four have pure covalent bond and are completely non-polar molecules. But as we move down the periodic table the polarizability increases and thus the dispersion forces increase.