We lump together the attractive forces between molecules, atoms, and ions as intermolecular forces (IMF). IMF is a general term for the attractions between discrete chemical units that we toss together under the general term "molecular."
All of these forces are driven by electrostatics, the forces felt between charged particles. In molecules these forces arise due to the electrons in the molecules.
We have three broad types of IMFs that we will deal with.
Dipole-Dipole forces. These are forces between polar molecules (a molecule with a permanent dipole moment, μ).
Hydrogen Bonding. This is a special case of extreme dipole-dipole forces that occurs when a hydrogen atom is bonded to a highly electronegative atom. The highly electronegative atom has to be N, O, or F.
Dispersion Forces. This one gets several names: Induced dipole-induced dipole, Dispersion forces, London forces, or van der waals forces. Dispersion Forces are often called the "weakest" but it is actually the most important since it is ubiquitous. Every molecule has dispersion forces. Not only do they have these forces, but they can add up across the whole molecule to be substantial. The degree to which a molecule has dispersion forces is measured via its polarizability, α. Polarizability (α) is to non-polar compounds, what dipole moment (μ) is to polar compounds.
Below is a summary of the range of IMFs as they relate to covalent bonds
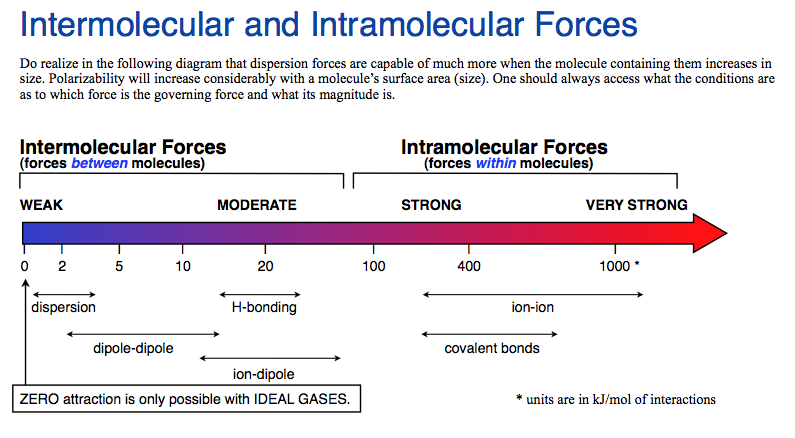
Here is a pdf of this diagram as a helpsheet.
Here is a video of Dr. McCord discussing intermolecular forces.
Intermolecular Forces