
There are a number of key liquid properties that can be understood based upon molecular structure (shape) and intermolecular forces. It is important to realize that for many compounds it is difficult to make comparisons between very different types of compounds. However, it is always possible to understand trends among similar compounds. For example, comparing CCl4 to water is a challenge since they have little in common. Are the dispersion forces from the highly polarizable Cl atoms more important than the hydrogen bonding to predict boiling point (vapor pressure)? This is hard to say without other information. In contrast, when comparing CH3OH to C2H5OH it is reasonable to assume they have similar hydrogen bonding but that the dispersion forces for the larger molecule will be larger.
Vapor pressure is a liquid property related to evaporation. In the liquid (or any substance) the molecules have a distribution of kinetic energies related to the temperature of the system. Because this is a distribution there will always be a few molecules that have enough kinetic energy to over come the attractive potential energy of the other molecules (the intermolecular force), and escape the liquid into the gas phase. In an open container, these molecules will wander off (diffuse) into the room and out into the atmosphere. Eventually all the liquid will evaporate.
In a closed container, the molecules that evaporate will diffuse around in the gas phase, but eventually some of them collide with the liquid. The kinetic energy of the gas molecules has the same distribution as the liquid because they are the same temperature. So now many of the molecules that collide with the liquid will not have sufficient kinetic energy to overcome the IMF and they will "stick" to the liquid. Therefore in a closed container, molecules are both evaporating (turning into gas) and condensing (turning into liquid). The rate of evaporation depends on the temperature and the IMF. The rate of condensation depends on the temperature, the IMF, and the concentration in the gas phase. The condensation rate will initially be zero since there are no molecules in the gas phase. As the evaporation continues the concentration of the molecules in the gas phase (or partial pressure) will increase. This will cause the rate of condensation to increase. When the rate of condensation is equal to the rate of evaporation, the system will no longer change and you'll have a fixed concentration in the gas phase. This concentration can be characterized as a partial pressure. That partial pressure is the "vapor pressure" (VP) of the liquid. The vapor pressure for any liquid then depends only on the temperature and the IMF. As temperature increases, VP increases. Stronger IMF mean that it requires more energy to overcome the IMF and enter the gas phase. Thus, the rate of evaporation will be lower and the VP will be lower. Conversely, weaker IMF will lead to higher VP.
While all liquids has some vapor pressure (VP),the VP is a strong function of the temperature. Therefore some liquid have a significant VP at room temperature while others do not. Liquids with a substantial vapor pressure at room temperature are call volatile. This means they will evaporate rapidly (within hours or days rather than weeks or months).
VP and boiling point (BP) are also related, as the boiling point is the temperature at which the VP is equal to 1 atm. Thus, the trend in BP with IMF is related to the trend of VP with IMF. High IMF = difficult to "get into" the gas phase. This equates to low VP (few molecules in the gas phase) and high boiling point (high temperature to get the VP = 1 atm).
Vapor PressureSurface tension is a property of liquids that arises due to the fact that the molecules at the surface of a liquid have a different potential energy than those in the bulk. Molecules that are at the surface are "missing" neighbors with whom they have attractive interactions (IMFs). As a result, they have a higher energy than molecules in the bulk that are surrounded by other molecules. Liquids will therefore minimize their surface area in order to minimize the number of higher energy molecules at a surface. Thus liquid drops will be spherical as a spherical shape has the minimum surface energy for a given volume (note: macroscopic drops are affected by gravity as well and thus have a teardrop shape). This effect also causes the surface to have a "tension." That is, one must apply a force in order to "break through" the surface. The easiest way to think about this is that by breaking or bending the surface you are always creating more surface molecules. This will cost some energy. So if the energy to break the surface is not sufficient, then the surface will not break. As a result, small objects will float on top of high surface tension liquids since the force down from the gravity on these objects will not be sufficient to overcome the surface tension of the liquid.
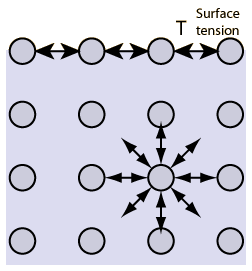
Above is a schematic of the molecular view of surface tension. The molecules at the surface have attractions for their neighbors but they don't have any molecules "above" them (these are the ones that are missing). Below is a picture demonstrating the macroscopic perspective of surface tension with a paper clip floating on water (despite the fact that metal in the paper clip has a higher density than water).
Viscosity is resistance to flow. For liquids, typically the larger the intermolecular forces (IMF) the higher the viscosity. The other factors that affect viscosity are temperature and the shape of the molecule. Higher temperatures will correspond to higher average kinetic energies and faster moving molecules. This will lead to a lower viscosity. The shape will also affect the viscosity, as molecules with many branches or kinks will be harder to "slide-by" one another than small "round" molecules.
Thus, carbon tetrachloride (CCl4) has boiling point of 350 K. Propanol (C3H7OH) has a boiling point of 370 K. You could conclude from this that they have similar IMF. CCl4 is tetrahedral (which is essentially spherical) while propanol is a carbon chain with a very sticky end. This means propanol is likely harder to flow due to its shape. Indeed the viscosity of propanol is about twice that or carbon tetrachloride.