
Surface tension is a property of liquids that arises due to the fact that the molecules at the surface of a liquid have a different potential energy than those in the bulk. Molecules that are at the surface are "missing" neighbors with whom they have attractive interactions (IMFs). As a result, they have a higher energy than molecules in the bulk that are surrounded by other molecules. Liquids will therefore minimize their surface area in order to minimize the number of higher energy molecules at a surface. Thus liquid drops will be spherical as a spherical shape has the minimum surface energy for a given volume (note: macroscopic drops are affected by gravity as well and thus have a teardrop shape). This effect also causes the surface to have a "tension." That is, one must apply a force in order to "break through" the surface. The easiest way to think about this is that by breaking or bending the surface you are always creating more surface molecules. This will cost some energy. So if the energy to break the surface is not sufficient, then the surface will not break. As a result, small objects will float on top of high surface tension liquids since the force down from the gravity on these objects will not be sufficient to overcome the surface tension of the liquid.
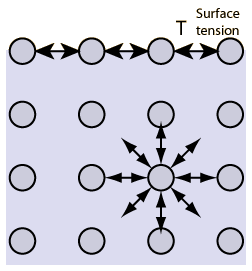
Above is a schematic of the molecular view of surface tension. The molecules at the surface have attractions for their neighbors but they don't have any molecules "above" them (these are the ones that are missing). Below is a picture demonstrating the macroscopic perspective of surface tension with a paper clip floating on water (despite the fact that metal in the paper clip has a higher density than water).