Electrons in covalent compounds are rarely exactly, equally "shared" by all the atoms. Rather the electrons have a greater relative attraction for some of the atoms compared to others. As a result, the electrons are not equally distributed throughout the molecule. This results in partial negative charges in some regions (those with a greater pull of electrons) and corresponding partial positive charges in the regions that are deficient in electrons. We determine where these charges are by comparing electronegativities.
The electronegativity of an element describes its relative "pull" of electrons in a covalent compound compared to other elements. High electronegativity elements will attract electrons more than low electronegativity elements.
The resulting covalent bonds then have a "polarity." That is to say one end is positive and one end is negative. This polarity is described quantitatively as a dipole moment. This is a measure of two charges separated by a particular distance.
Depending on the geometry of a molecule, the entire molecule may have a dipole moment. This is a quantity that we can measure in the lab as an observable. Molecules with large dipole moments we call "polar." Those with very small or no dipole we call "non-polar."
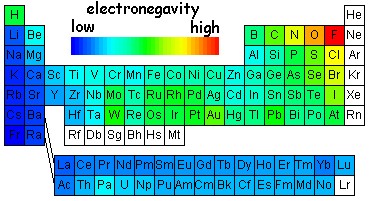
Electronegativity describes the relative pull of the a particular elements on the electrons in a covalent compound. There are several electronegativity scales that have been developed over the years to describe bonding. Some are based on elemental properties (ionization energy and electron affinity) other are based on bond strength measured in various compounds. However, all the scales share the same qualitative idea. The higher the electronegatively the greater the attraction the electrons have for that element. Since the scale is relative (a comparison between different atoms) F is typically chosen as the most electronegative atom and its electronegativity is set at 4.0. Electronegativity has a periodic trend that is nearly identical to that of ionization energy. It decreases top to bottom and it increases left to right. The reason behind this is nearly identical to that of ionization energy. The pull of the nucleus increases left to right and decreases top to bottom. Below is a scale of electronegativities. To calibrate the diagram F = 4.0 and Cs = 0.8

You can see a few exceptions to the strict increases left to right and decreases top to bottom (particularly in the transition elements). However in general, the upper right is the highest and the lower left is the lowest. Another notable outlier is hydrogen. Because hydrogen has only one electron, it is quite different from the other elements. Its electronegativity is nearly identical to that of carbon.
We often lump bonds into being either ionic or covalent. But this greatly simplifies what is again a complex quantum mechanical problem. Luckily our over simplification gives us the correct perspective much of the time. Nonetheless, it is important to realize that this actually is a spectrum of different types of bonds ranging from what we would call purely covalent to ionic. In between, bonds might be best described as partly ionic and partly covalent. For such compounds we talk about their "covalent character" or "ionic character." Thus NaCl is something in which we would say the bonding is dominated by the ionic character. We can qualitatively lump bonds into these different categories by differences in electronegativities. Large differences lead to ionic bonds (bonds with mostly ionic character) while small differences lead to covalent bonds (bonds with mostly covalent character). Before, we simply said ionic bonds form between a metal and a non-metal, and covalent between a non-metal and a non-metal. However, looking at electronegativity differences, you see that this is the same idea as non-metals have larger electronegativities than metals.
We then further divide up the covalent bonds into two categories. Pure covalent and polar covalent. Pure covalent are bonds between two elements with identical electronegativities. For these bonds, the electrons are perfectly equally shared between the two atoms. Polar covalent bonds form between elements with different electronegativities but those that aren't large enough that we could call the bonds ionic. For example, the bond between hydrogen and chlorine. Chlorine has a larger electronegativity so we would expect this bond to be polar, with more of the electrons residing on the chlorine. To denote this in a structure we would draw a small partial charge on the atoms using a lower case delta, δ, with a plus or a minus sign. The element with the higher electronegativity will have a greater share of the electrons and thus it will be the δ-. Note, the charges are partial. This implies that the electron spends more time "on that atom" but is not completely transferred (that would be the ionic picture).
A dipole moment is a quantity that describes two opposite charges separated by a distance. It is a quantity that we can measure for a molecule in the lab and thereby determine the size of the partial charges on the molecule (if we know the bond length). By definition the dipole moment, μ, is the product of the magnitude of the separated charge and the distance of the separation:
where \(q\) is the magnitude of the separated charge and \(r\) is the distance between them.
If we were to use SI units, charge would be in Coulombs and distances in meters. However, the charges were are talking about in molecules are very small (partial electron charges) and the distances are tiny as well (less than 1 nm). So this would lead to dipoles that are very, very small. So instead we use another historical unit, the Debye. 1 Debye is approximately 3.33 x 10-30 C*m. Molecules typically have dipole moments around 1 D.
If a molecule has a dipole moment, then we call it "polar." However, how big does the dipole have to be? Can it be 0.0001 D? That is ten thousand times less than a molecule with a dipole of 1 D. There is no strict cutoff. Nonetheless, molecules with atoms with very small electronegativities differences typically will have bonds that are only very slightly polar. This will lead to overall dipole moments for the molecule that are very, very small and would be considered non-polar. The most important example of this is hydrocarbons, molecules that contain hydrogen and carbon. While H and C don't have identical electronegativities, they are very close. So the C-H bond is very, very weakly polar. Overall, chemists (and biologists) would consider hydrocarbons to be non-polar (even though technically they might have very tiny dipole moments).
Most important when determining if a molecule has a dipole moment are two factors. One it must have polar covalent bonds. Two, it must have a shape in which all the dipoles don't cancel. Thus, our great interest in the shapes of different molecules.