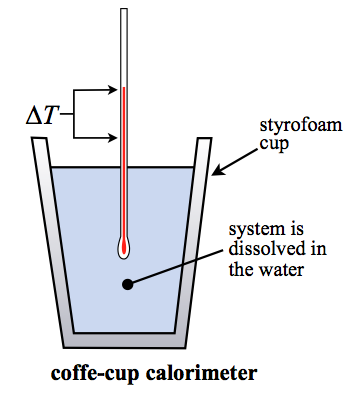
A coffee cup calorimeter is a constant pressure calorimeter. As such, the heat that is measured in such a device is equivalent to the change in enthalpy. A coffee cup calorimeter is typically used for solution based chemistry and as such generally involves a reaction with little or no volume change. Therefore, while the heat is exactly equal to the enthalpy change (\(\Delta H\)) in this case, it will also be approximately equal to the change in the internal energy (\(\Delta U\)) since the work will be very small (assuming there are no gas reactants or products).
The more technical name for this type of calorimetry is isobaric calorimetry. The "coffee-cup" name comes from the fact that most of the time this experiment is done inside of a simple styrofoam cup. A styrofoam cup makes for a good adiabatic wall and helps keep all the heat released or absorbed by the reaction inside the cup so we can measure it.
The heat capacity of a coffee cup calorimeter is typically taken to be that of the water in the calorimeter. However, it could be that the "hardware" and the water heat capacities have both been measured and you treat it much like in bomb calorimetry. None-the-less, most coffee-cup calorimeters have virtually no hardware (it's a styrofoam cup!) and you can ignore that component. So the equation of interest is:
\[q_{\rm cal} = m_{\rm water} C_{\rm s,water}\Delta T = -q_{\rm p,system} \]
Note that for such a measurement, the heat of the reaction is assumed to all go into the temperature change of the water. This is why the heat can be easily quantified using the mass of the water, the specific heat capacity of the water, and the temperature change. Since this measurement is at constant pressure (isobaric), the heat is equal to the change in enthalpy or \(\Delta H\). Note: be careful with the sign on \(\Delta H\). Heat out of the reaction is heat in to the calorimeter and vice versa. So an increase in temperature means the reaction was "exothermic" and \(\Delta H\) is negative. Conversely, if the temperature drops during the reaction, then the reaction must be endothermic and \(\Delta H\) is positive.