The formal charge is an idea of accounting for the distribution of electrons in an atom. This can help in two ways.
1. It can help us decide which of several Lewis dot structures is closest to representing the properties of the real compound.
2. It can help us envision where there might be regions of positive or negative charge in a molecule.
First, how does it help to decide between different structures? Our general rule is that the best structure minimizes the formal charges. This is because minimizing the formal charges leads to the electrons being most evenly distributed about the different atomic centers in a molecule. Having electrons concentrated in one area will lead to regions of negative charge. The atoms that are now "missing" electrons will be positive in charge. Separating positive and negative charges costs energy and thus we conclude that the lowest energy (best structure) would minimize having separated charges.
How do we find these charges? We look at how many valence electrons the atoms "has" in the molecule compared to how many it has on its own. It is important to know that this is a very general idea that grossly over simplifies the quantum mechanics. The electrons in a molecule have no memory of where they came from or to which atom they "belong". They are simply spread throughout the molecule. None the less, these simple ideas can help us to arrive at the best structures as well as understand something about the charge distributions.
In a molecule, we assign each atom a formal charge. This charge is the number of electrons it had as valence electrons minus the number it "has" in the molecule. The number it has in the molecule is a combination of the lone pair electrons and the shared bonding electrons. For each atom, we will count all of the lone pair electrons but only half of the bonded electrons (as they are shared). This is easiest to account for by just counting the number of bonds. So the formal charge is
Formal charge = Valence Electrons - [Lone Pair Electrons + (# of Bonds)]

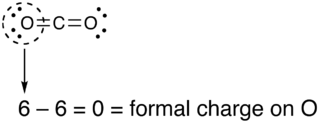
So for example if we look at CO2 each oxygen has two lone pairs (4 electrons) and 2 bonds (double bond). Oxygen has 6 valence electrons. So the formal charge on each oxygen atom will be 6-(4+2)=0.
You need to find the formal charge on each atom in a compound. However, there are some short cuts that will help. The sum of all the formal charges must be the charge on the molecule. For neutral compounds this will be zero. Since each oxygen in CO2 is zero, and the total charge is zero, the formal charge on carbon must be zero.