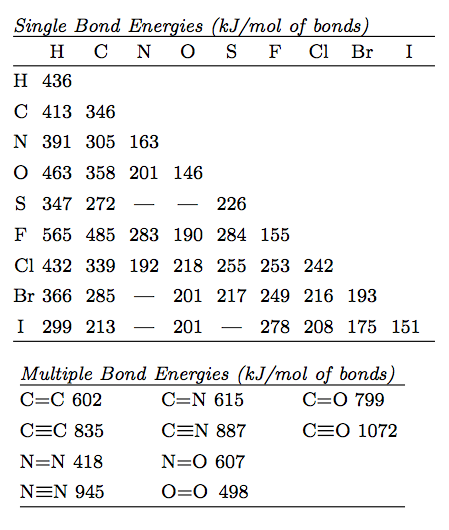
The heat associated with a reaction is the result of bonding breaking and bond formation. This is, after all, what a chemical reaction is. Given the typical energies of different types of bonds we can estimate the enthalpy change for any given reaction. The energy will be the total of the energy required to break any bonds that are broken and the energy released from any bonds that are formed. This is the sum of the bond enthalpies of the broken bonds minus the sum of the energies of the bonds that are formed (since forming bonds will release energy). One advantage of this method is that you only need to look at the bonds that are changing in a given reaction.
Consider the hydrogenation of ethene to give ethane.
\[\rm C_2 H_4 (g) + H_2 (g) \rightarrow C_2 H_6 (g)\]
Here, we need to break the C=C bond in ethene, and the H-H bond in H2. Then we need to form two new C-H bonds and a C-C bond in ethane. (See Bond Energy table at the bottom of this page) A H-H bond enthalpy (BE) is 436 kJ mol-1, a C=C bond is 602 kJ mol-1, a C-C bond is 346 kJ mol-1, and a C-H BE is 413 kJ mol-1. All of the changing energies for the reactants and products for this reaction are shown in the table below.
| reactants | ΔH | products | ΔH |
| break 1 C=C @ 602 = | 602 | make 1 C-C @ 346 = | 346 |
| break 1 H-H @ 436 = | 436 | make 2 C-H @ 413 = | 826 |
| total breaking | 1038 | total making | 1172 |
Because the bonds in the reactants are broken (energy in, endothermic, ADD the amounts) and the bonds in the products are formed (energy out, exothermic, SUBTRACT the amounts), you can also calculate the enthalpy by breaking all the reactant bonds and forming all the product bonds. This results in the change in enthalpy being the sum of the all the reactant bond enthalpies minus the sum of all the product bond enthalpies.
\[ \Delta H_r = \Sigma BE_{reactant} - \Sigma BE_{products}\]
Sometimes this is very lengthy so an alternative is to just look at the bonds that are changing as in the example above.
For this method you need a table of bond enthalpies like the one given below. Please note that the top half is for single bonds and the bottom half is for double and triple bonds. Make sure you have the right bond for your specific compound(s).